We’re pleased to introduce DAXXIFY™–the only long-lasting, peptide-powered frown line treatment. We’re one of the first exclusive providers to offer this innovative treatment. Call our office today to book your appointment and visit DAXXIFY.com to
learn more.
What is DAXXIFY™ and how is it different?
DAXXIFY™ is FDA approved to help smooth moderate to severe lines between the brows. It is the only long-lasting frown line treatment powered by a peptide with results that last on average 6 months and up to 9 months for some.*
Conventional injectables last 3 – 4 months and require up to 4 treatments a year to maintain results.
Nearly 90% of patients say they wish results lasted longer. With DAXXIFY™ you can keep your frown lines smoother for a full year with as few as 2 treatments.*
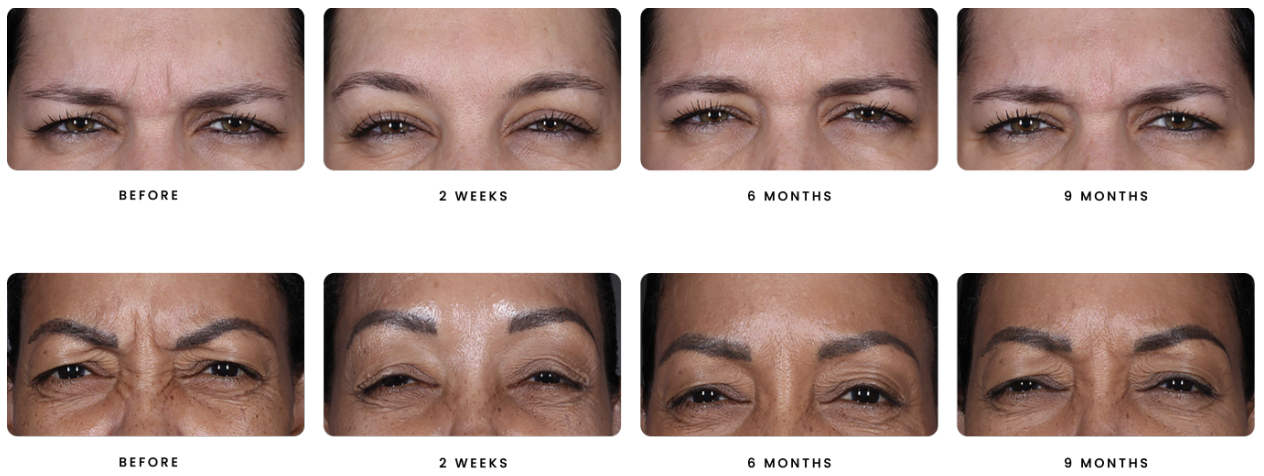
*At least 50% of patients in clinical studies had no or minor frown lines 6 months after treatment.
Between 3% and 5% of patients in clinical studies had no or minor frown lines 9 months after treatment.
In other safety studies, between 5% and 17% of patients still had noticeable improvement 9 months later
Why are peptides important?
All frown line treatments require a special ingredient to stabilize botulinum toxin A, the protein responsible for helping smooth moderate to severe frown lines. For instance, BOTOX® Cosmetic uses human serum albumin (HSA), a blood product, as its stabilizer. Dysport®, another frown line treatment, uses HSA as a stabilizer and cow’s milk protein as a protectant.
DAXXIFY™ is unique because it is the only formulation that uses a novel peptide as a stabilizer and does not contain human or animal byproducts.
Is DAXXIFY™ well studied?
DAXXIFY™ was studied in the largest-ever clinical study conducted for a frown line treatment and included more than 2,400 people across different ages and skin types.
- There were no serious treatment-related side effects in clinical trials for DAXXIFY™
- The active ingredient in DAXXIFY™ is botulinum toxin type A, an ingredient that has been used in frown line treatments for more than 20 years
- 96% of people treated with DAXXIFY™ were satisfied with their results†
† 96% of patients reported they were satisfied on a 7-point scale when evaluated at 4 weeks in clinical studies.
IMPORTANT SAFETY INFORMATION for DAXXIFY™ (daxibotulinumtoxinA-Ianm) injection
DAXXIFY™ may cause serious side effects that can be life threatening. Get medical help right away if you have any of these problems any time (hours to weeks) after injection of DAXXIFY™
- Problems swallowing, speaking, or breathing due to weakening of associated muscles can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection.
Swallowing problems may last for several months. - Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms that include loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, and trouble swallowing.
- Do not receive DAXXIFY™ if you are allergic to any of the ingredients in DAXXIFY™ (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as rimabotulinumtoxinB (MYOBLOC®), onabotulinumtoxinA (BOTOX®/BOTOX® Cosmetic), abobotulinumtoxinA (DYSPORT®), incobotulinumtoxinA (XEOMIN®) or prabotulinumtoxinA-xvfs (JEUVEAU®); or have a skin infection at the planned injection site.
DAXXIFY™ dosing units are not the same as, or comparable to, any other botulinum toxin product.
Tell your healthcare provider about all your medical conditions, including any side effects from botulinum toxin products, including dry eye; breathing, swallowing, bleeding, or heart problems; plans to have surgery; weakness of forehead muscles; drooping eyelids; have had surgery on your face; are pregnant or breastfeeding or plan to become pregnant or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-thecounter medicines, vitamins, and herbal supplements.
Using DAXXIFY™ with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your healthcare provider that you have received DAXXIFY™ in the past.
Especially tell your healthcare provider if you have received any other botulinum toxin product in the last 4 months or any in the past, and exactly which product you received (such as BOTOX®, BOTOX® Cosmetic, MYOBLOC®, DYSPORT®, XEOMIN®, or JEUVEAU®). DAXXIFY™ may cause serious side effects, including allergic reactions (such as itching, rash, redness, swelling, wheezing, trouble breathing, or dizziness or feeling faint), heart problems (such as irregular heartbeat and heart attack), and eye problems (including dry eye, reduced blinking, and corneal problems). Tell your healthcare provider or get medical help right away if you experience a serious side effect. No serious adverse events of distant spread of toxin effect associated with dermatologic use of DAXXIFY™ have been reported in clinical studies at the dose of 40 Units for glabellar lines. The most common side effects of DAXXIFY™ include headache, eyelid drooping, and loss of ability to move the muscles in your face.
These are not all the possible side effects of DAXXIFY™. For more information, see the full Prescribing Information including Boxed Warning, and refer to the Medication Guide or talk with your doctor. To report side effects associated with DAXXIFY™, please call 1-877-373-8669. You may also report side effects to the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
APPROVED USE
DAXXIFY™ is a prescription medicine that is injected into muscles and used in adults to temporarily improve the look of moderate to severe frown lines between the eyebrows.
DAXI-001723
Zel Skin & Laser Specialists – Plymouth ★★★★★ on Google5:27pm
Felt COvid safe ... always a great experience working with professionals
Brian brings a unique combination of medical science, clinical expertise, integrity and compassion to his patients. He provides the latest technology available in the field of dermatology but with a personalized touch for each patient. He is highly respected by his colleagues throughout the country for his expertise and sincerity.
Everyone I have met at Zel is amazing!
It is rare to find a dermatologist who is as knowledgeable about the many facets of clinical and cosmetic dermatology as Dr. Zelickson. Because of his knowledge and honesty, doctors worldwide know they can depend on his assessment of new therapies, procedures and medical devices. This, in turn, has made him a much sought–after speaker for the plethora of professional medical society meetings and engagements that we have throughout the year. I am honored to know Dr. Zelickson and consider him a friend to the profession.
Dr. Brian Zelickson was one of our initial Thermage investigators and helped us understand the mechanisms of non–invasive skin tightening. He is a renowned expert on how collagen can affect skin tightening and wrinkle reduction.
Dr. Brian Zelickson is one of this country’s leading laser experts. Not only is he unparalleled as a laser surgeon, it is not an exaggeration to state that his superb research helps determine the future of dermatologic laser surgery.
Dr. Zelickson is a highly regarded skin/light expert in our industry. When Candela looks for both medical and scientific advice for new laser applications, we often turn to him for his counsel. The input he provides us always starts with patient safety and satisfaction. We feel fortunate to be able to tap into Brian as an independent resource. The patients who are treated with Candela devices are often the beneficiaries of Brian’s input to us.
Most patients prefer seeing a doctor who is known for a particular specialty or procedure, but all patients want to see the doctor who invented the procedure or therapy. Dr. Brian Zelickson is one of the most respected physicians in the field of cosmetic dermatology and aesthetic medicine. He has lectured around the world and is one of the most sought–after speakers for all major meetings in dermatology.
I would rate Dr. Zelickson's clinic 10 stars!
We have felt honored to have Dr. Brian Zelickson, a world–renowned dermatologist who is distinguished as a high–quality care physician, serving as the investigator evaluating Syneron non–invasive skin tightening technology
I trained with Dr. Zelickson at Mayo Clinic, and I found him to be a very bright and creative dermatologist and scientist. Later, he was our best advisor when we set up our clinic in the Basque Country in Spain. I call him the ‘paparazzi of dermatology.’ His contribution to our speciality is probably among the best of this century.
Tuve la suerte de conocer al Dr. Brian Zelickson en Minneapolis hace muchos anos y desde entonces acudo regularmente a compartir sus conocimientos. Es un científico honrado, brillante, creativo e innovador. Nunca deja de sorprenderte agradablemente.
Dr. Zelickson is a very well–respected dermatologist, not only in the U.S., but also in Europe. He is famous for being the author of relevant dermatological studies published in many prestigious medical journals and for the improvements he’s made on minimally invasive cosmetic procedures that are being used by dermatologists all over the world.
Our view from Europe of Dr. Brian Zelickson is that he is one of the leading four or five U.S. laser dermatologists. His research contributions, his superb lectures and his expert knowledge of laser skin surgery make him a valued friend, colleague and guest.
Dr. Brian Zelickson is one of the world’s leaders in cutaneous laser surgery. He has performed groundbreaking research over the past decade, advancing the field as well as expertly providing the most up–to–date laser treatments for his patients.
Brian Zelickson is a world–renowned laser expert who helps develop the lasers and treatment parameters used by the rest of the world. He is where others turn to learn the latest techniques in laser surgery. If you want the best treatment available, delivering the best results in the safest environment available, there is no need to look anywhere else.
I have met many laser experts in my 20 years of teaching, lecturing and running a busy practice. There is no one who I respect more than Brian. If I have a clinical question or research idea, Brian is one of the few individuals I call, as I know I can count on his intellect, integrity and common sense.
Dr. Zelickson is one of America’s most knowledgeable laser experts. He has done pioneering work with many of the laser and light–based technologies developed in the world over the last 10 years. He is a giant in the field.
Dr. Zelickson is one of the world’s leaders in the field of lasers for the treatment of the skin. His many years experience in treating thousands of patients with lasers and other medical devices makes him one of the most sought–after doctors in this field. Innovation and caring are his trademarks.
